 |
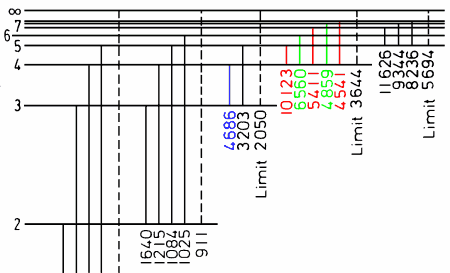
| He II: Energy levels of singly ionized Helium. Pickering series of lines is marked in red. Photoionization thresholds are marked in dashed lines (Series Limits). The five transitions to the ground (n=1) level are not labeled. |
 |
| He II: Energy levels of singly ionized Helium. Pickering series of lines is marked in red. Photoionization thresholds are marked in dashed lines (Series Limits). The five transitions to the ground (n=1) level are not labeled. |
The transitions between the singly ionized helium energy levels corresponding to the Pickering series are 4-5 (10124 Å), 4-7 (5412 Å) and 4-9 (4522 Å) (red in above diagram). Note that the 4-6 (6560 Å) and 4-8 (4859 Å) (green in diagram) transitions were originally not included in this series because they coincided with the hydrogen series of lines and were thus obscured (except in hydrogen deficient stars). Although the 3-4 (4686 Å) (blue in diagram) transition also belongs to ionized helium and often occurs in these hot stars, it did not belong to the pickering series because it has a lower landing level quantum number (n=3 instead of n=4).
In 1912, Pickering still believed it was hydrogen, even when one of Lockyer's pupils, Alfred Fowler showed that these lines are produced in a laboratory mixture of hydrogen and helium. This unusual oversight could partially be attributed to the fact that Fowler still subscribed to Pickering's belief that it was hydrogen. (Just another example that belief is stronger than reason !).
At the turn of the century the physicist Max Planck discovered energy quantization in his theoretical analysis of black body radiation. Albert Einstein later established that these energy quanta are photons or particles of light. Then came Rutherford's model of the atom made up of small massive positively charged nucleus surrounded by a relatively large volume occupied by the negatively charged electrons.
 The physicist
Niels Bohr,
founding father of quantum mechanics, synthesized all
these separate facts into a coherent picture of the atom in which the electron orbits
were quantized into stationary states and exchange energy with the electromagnetic
field in discrete energy packets called photons. His theory predicted the spectrum
of singly ionized Helium which corresponded exactly to the Pickering line series.
The helium nuclei has twice the charge of the hydrogen nuclei, this gave rise to
Pickering's spurious half-integral quantum numbers.
The physicist
Niels Bohr,
founding father of quantum mechanics, synthesized all
these separate facts into a coherent picture of the atom in which the electron orbits
were quantized into stationary states and exchange energy with the electromagnetic
field in discrete energy packets called photons. His theory predicted the spectrum
of singly ionized Helium which corresponded exactly to the Pickering line series.
The helium nuclei has twice the charge of the hydrogen nuclei, this gave rise to
Pickering's spurious half-integral quantum numbers.